16. Regulatory Landscape
Regulatory Landscape
ND320 C2 L1 18 Understanding Key Stakeholders - Clinical
ND320 C2 L1 19 Understanding Key Stakeholders - Industry
ND320 C2 L1 20 Understanding Key Stakeholders - Regulatory
Typo in the slide: should be implantable pacemaker
Summary
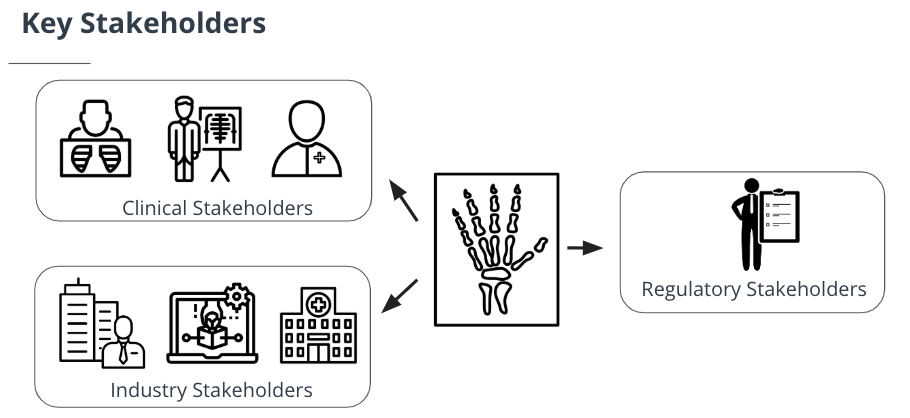
Key Stakeholders
Clinical Stakeholders
Clinical stakeholders are radiologists, diagnosing clinicians and patients. Radiologists are likely the end-users of an AI application for 2D imaging. They care about low disruption to workflow and they play an important advisory role in the algorithm development process. Clinicians have less visibility into the inner workings of an algorithm. They also care about low disruption to workflows and they care about the interpretability of algorithm output. Patients may be the most important stakeholder, and the FDA looks at your algorithm through the lens of protecting the patient from all unnecessary risks. Patients may never know that AI is involved and they care about the timeliness of receiving accurate test results.
Industry stakeholders
Industry stakeholders include medical device companies, software companies, and hospitals. Many medical device companies typically have accompanying imaging software. They also build their own AI algorithms to run on their hardware. Software companies can act more dynamically because they are not tied to a specific hardware system, but this also poses a regulatory challenge as the FDA wants to know if an algorithm performs the same across all hardware systems, and if not, which ones it is not appropriate for. Hospitals must be sure that they have the adequate infrastructure needed for algorithm deployment. In order to purchase an algorithm, a hospital must be convinced that it will save them money in the long run.
Regulatory stakeholder
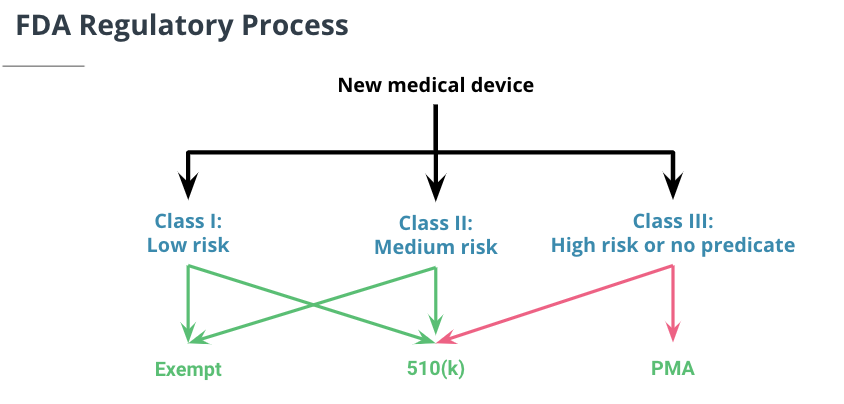
The main regulatory stakeholder in the medical imaging world is the Food and Drug Administration (FDA). The FDA treats AI algorithms as medical devices. Medical devices are broken down into three classes by the FDA, Class I, Class II, and Class III, based on their potential risks present to the patient. A device's class dictates the safety controls, which in turn dictates which regulatory pathway they must go down. The two main regulatory pathways for medical devices are 510(k) and Pre-market Approval (PMA). Lower risk devices (Classes I & II) usually take a 501(k) submission pathway. Higher risk devices and algorithms (Class III) must go through PMA.